Draft Medical Representative Measures: Challenges & Mitigations
Draft Medical Representative Measures: Challenges & Mitigations
On November 28, 2024, the National Medical Products Administration (“NMPA”) issued the Administrative Measures on Medical Representatives (Draft for Comments) (“2024 Draft Measures”) to solicit public comments. If the 2024 Draft Measures takes effect, the widely adopted approach of outsourcing medical representative teams to contract sales organizations (“CSOs”) will be banned, and the threshold for becoming medical representatives will be raised. If these changes become effective, they will present great challenges to pharmaceutical companies in China.
1、A Retrospective Look at China’s Regulation of Medical Representatives
a.Early Days: Introduction of Medical Representatives and Initial Regulations
The emergence of medical representatives in China can be traced back to the late 20th century when foreign pharmaceutical companies introduced the role amid the nation’s efforts to modernize its economy and healthcare system. Later on, as incidents of corruption among medical representatives escalated, Chinese regulatory authorities recognized in 2006 the need to address unethical practices in this thriving profession. In the following decade, some regions experimented with regulations to manage interactions between healthcare professionals (“HCPs”) and medical representatives, but the absence of a cohesive regulatory framework caused the oversight of medical representatives to remain underdeveloped.
b.The Turning Point: Kickback Scandals and Regulatory Reform
In late 2016, CCTV-13, China’s state-owned news channel, aired a program titled High Drug Prices Driven by High Kickbacks. The investigative report revealed that hundreds of medical representatives frequented hospitals daily, accessing consulting rooms, reading prescription data on computers, and collecting medication information. These medical representatives were found to offer HCPs “red envelopes” containing kickbacks amounting to approximately 35% of the drug’s price, in return for higher prescription volumes. The shocking exposé drew nationwide scrutiny, positioning medical representatives at the epicenter of the public controversy.
In response, the State Council issued the Several Opinions on Further Reforming and Improving of Policies for the Production, Circulation, and Use of Pharmaceuticals in early 2017(commonly known as the “17 State-Pharmaceutical-Policy Articles”, as this policy contains 17 articles). This policy explicitly calls for (i) establishing a national filing system for medical representatives, (ii) limiting their activities to academic promotions only, and (iii) prohibiting their involvement in drug sales.
To enforce the 17 State-Pharmaceutical-Policy Articles, the National Food and Drug Administration (the predecessor of NMPA) issued (i) the Administrative Measures for the Registration and Filing of Medical Representatives (Tentative) (Draft for Comments) in 2017 (the “2017 Draft Measures”), and (ii) the Administrative Measures for the Filing of Medical Representatives (Tentative) (Draft for Comments) in 2020 (the “2020 Draft Measures”) to solicit public comments on the regulations of medical representatives.
In September 2020, the Administrative Measures for the Filing of Medical Representatives (Tentative) (the “2020 Tentative Measures”) was officially promulgated. Since then, all medical representatives have been required to file their information with the NMPA’s filing platform.
c.Escalation: Anti-Corruption Campaign and Stricter Supervision
In 2023, China launched an anti-corruption campaign targeting the medical and pharmaceutical sectors. As a result, numerous medical representatives as well as HCPs faced administrative or criminal penalties for their bribery-related offenses, and most academic conferences targeting HCPs were suspended.
In May 2024, the National Health Commission (“NHC”), together with 13 other law enforcement agencies, called for stricter regulatory measures. Against this backdrop, the 2024 Draft Measures was released in November 2024, which adopted tighter supervision measures on medical representatives to further combat corruption.
2、Key Points of the 2024 Draft Measures
a.Prohibition on Outsourcing Medical Representatives
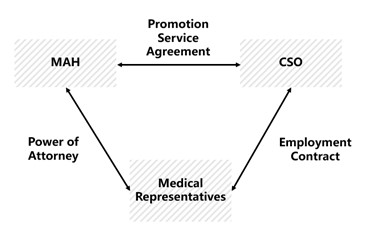
Under the 2020 Tentative Measures, a Marketing Authorization Holder (“MAH”) can either (i) directly hire medical representatives, or (ii) outsource them to a CSO. Under option (ii), the arrangement works as follows:
(1)The CSO signs employment contracts with medical representatives;
(2)The MAH signs promotion service agreements with the CSO; and
(3)The MAH issues a power of attorney to CSO’s medical representatives, authorizing them to carry out promotional activities on behalf of the MAH.
It can be illustrated as follows:
Under the 2024 Draft Measures, however, outsourcing medical representatives is no longer permitted. Article 9 of the 2024 Draft Measures stipulates that “when an MAH carries out academic promotion activities for drugs, it shall employ a medical representative. The MAH shall sign an employment contract and a compliance commitment letter with the medical representative. If the medical representative violates the compliance commitment, the employment contract shall be terminated in accordance with the law.” The employment contract is required to be filed with the filing platform.
In other words, under the 2024 Draft Measures, only medical representatives directly employed by an MAH are allowed to engage in medical promotion activities. Outsourcing medical representatives to a CSO will be banned.
b.Raising the Qualification Bar for Medical Representatives
The 2017 Draft Measures required that medical representatives must either (i) hold a higher vocational diploma, a junior college diploma, or a higher-level diploma in a field related to pharmaceuticals, or (ii) hold a higher vocational diploma, a junior college diploma, or a higher-level diploma in fields other than pharmaceuticals, complemented by more than two years of pharmaceutical-related work experience. However, this requirement was removed in the 2020 Draft Measures and was not adopted in the 2020 Tentative Measures.
The 2024 Draft Measures proposes much higher requirements by stipulating that medical representatives should (i) hold a college or higher-level diploma in a pharmaceutical-related field, or alternatively, hold a mid-or-higher level professional technical title, (ii) possess clinical theoretical knowledge and practical drug experience or relevant work experience in drug R&D, production, inspection, quality management, or other related fields, (iii) thoroughly understand the drugs being promoted, and (iv) have completed the training provided by the relevant MAH and passed the corresponding assessment.
This elevated threshold aims to (i) screen out medical representatives who lack adequate pharmaceutical expertise, and (ii) steer medical representatives’ focus from sales promotion back to the core mission they are supposed to focus on, that is, to professionally and accurately introduce and disseminate drug information on behalf of MAHs.
c.Registration with Hospitals
Since the 2020 Tentative Measures took force, medical representatives have been required to file their information with the NMPA’s filing platform. However, this scheme has a loophole, as it is difficult for hospitals to verify whether a medical representative has indeed completed the filing.
The 2024 Draft Measures aims to address the loophole by requiring medical representatives to not only file their information with the NMPA filing platform but also register with each hospital. Hospitals and HCPs may only interact with medical representatives who have completed both the NMPA filing and the hospital registration.
d.Prohibited Acts
The 2024 Draft Measures clearly specifies the acts prohibited for MAHs, medical representatives, and hospitals, which are summarized as follows:
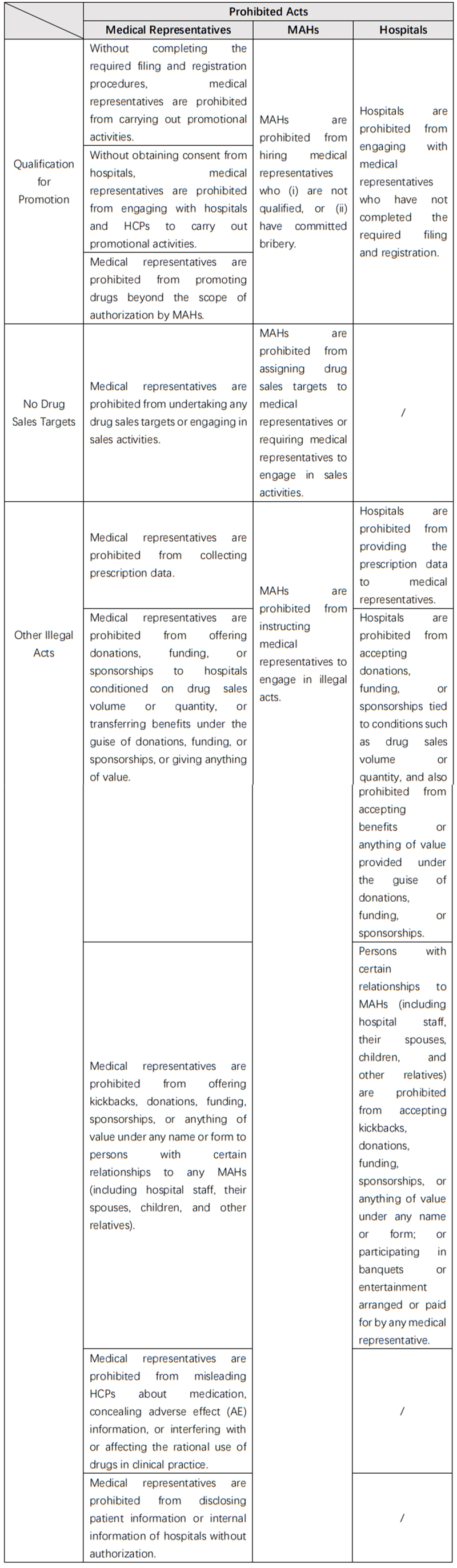
To ensure strict enforcement of these prohibitions, the 2024 Draft Measures confers regulatory authority upon multiple government agencies (including but not limited to NMPA and NHC) to oversee the compliance by stakeholders. Medical representatives, MAHs, and hospitals engaging in these prohibited acts would face penalties imposed by these authorities.
3、Mitigation Measures
Once the 2024 Draft Measures takes effect, pharmaceutical companies are anticipated to face substantial impacts. To avoid being caught unprepared, companies should proactively develop comprehensive response plans. Key strategies include the following:
1、Review and Revise CSO Promotion Agreements. Pharmaceutical companies should evaluate existing agreements with CSOs and plan for necessary modifications or terminations. Under the 2024 Draft Measures, CSOs will no longer be permitted to directly interact with HCPs. Their roles may be restricted to designing promotional strategies, organizing academic conferences, and preparing marketing materials. As a result, agreements that previously allowed direct engagement with HCPs will require adjustments to align with the new regulatory framework.
2、Build an In-House Team of Medical Representatives. Companies that have relied heavily on outsourcing medical representatives to reduce personnel management costs should gradually transition to establishing high-quality in-house teams. Downsizing outsourced teams and focusing on hiring skilled, in-house medical representatives will be essential to comply with the new requirements and maintain effective promotional activities.
3、Develop a Robust Compliance System for Medical Representatives. Companies should implement a comprehensive compliance framework to address the new regulatory standards. This may include (i) establishing clear criteria and procedures for selecting and employing medical representatives; (ii) ensuring timely fulfillment of registration and filing obligations; (ii) conducting internal training programs and performance assessments for all medical representatives; (iii) creating a standard code of conduct for professional visits by in-house representatives; and (iv) setting up a whistleblowing mechanism and internal disciplinary measures to address potential violations.
Regardless of how the new regulations are enforced, they will present significant challenges to pharmaceutical companies. By planning and preparing in advance, as well as establishing a robust compliance system, companies can position themselves for long-term success and operation stability in this evolving regulatory landscape.









